NSTDA held a press conference on 19 April 2019 announcing the transfer of M-Bone, a hydroxyapatite bone graft substitute technology, to Oss Hydroxy Company Limited, a startup in Thailand specializing in the manufacturing and distribution of medical supplies and devices. The licensing agreement allows Oss Hydroxy to manufacture and commercialize M-Bone for a duration of 5 years.

M-Bone is a synthetic bone graft material made of hydroxyapatite and tricalcium phosphate as major components. It was developed by Dr. Naruporn Monmaturapoj, Senior Researcher of NSTDA Assistive Technology and Medical Devices Center (A-MED). M-Bone has been tested extensively for toxicity, biocompatibility, postoperative fever and side effects on patients, and the ability to induce bone regeneration. In the evaluation for its application in dental implant, M-Bone could induce the new bone cells to form in just 4 weeks after the implant. By week 16, the natural bone could be seen growing into the pores of bone graft whereas the synthetic bone slowly disintegrated. Within 3 months, the new natural bone was fully formed and completely covered the entire space. It normally takes 6 months for new bone to fully form for ordinary patients, and even 1 year in the case of the elderly. Unlike bone graft harvested from humans or animals, the synthetic M-Bone is sterile, and thus eliminates the risk of disease transmission.
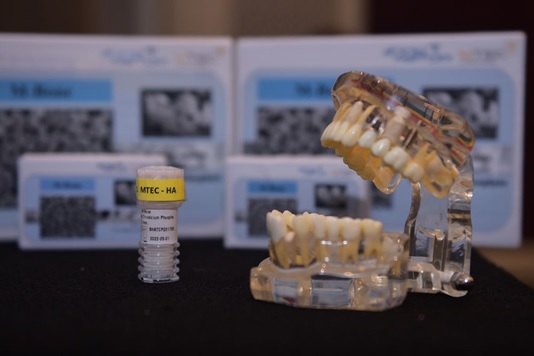
In the initial stage, NSTDA will manufacture M-Bone for Oss Hydroxy Company Limited to launch the marketing campaign. The company plans to establish its own manufacturing facility in 2020.
NSTDA’s M-Bone manufacturing facility is designed to comply with ISO 13485:2016 requirements for manufacturing medical devices and supplies, and has successfully been registered as a medical device manufacturing establishment with the Thai Food and Drug Administration.
Demand for synthetic bone material is growing due to the increase in aging population and improved healthcare system. The material is costly, around 3000 -4000 baht per 0.5 cc. as it needs to be imported. The transfer of this technology to a local company will enable the domestic production of this medical supply and make it more affordable.
